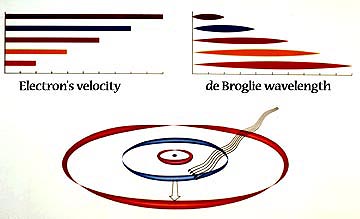
|
Ernest Rutherford
(1871-1937) worked with radioactivity, which he found to be of
different kinds, distinguishing between positive, or alpha rays, and
negative, or beta rays. In this work he was assisted by the German
physicist Hans Geiger , with whom he devised an instrument to detect
and count these particles. The instrument consisted of a tube
containing gas with a wire at high voltage along the axis. Each
particle entering the tube ionized some gas, causing an electron
avalance and a measurable pulse, or click. He discovered that the alpha
particles were helium nuclei. By 1909, Geiger and E Marsden used alpha
particles to bombard very thin metal foils. Sometimes the alpha
particles were scattered backward as if repelled by other positive
charges; this led Rutherford to propose the theory of a nuclear atom,
containing a very small positive nucleus surrounded by negatively
charged electrons and a great deal of empty space. In 1928 Geiger and
W. Muller designed a improved counter to measure radioactivity, the
Geiger- Muller counter.



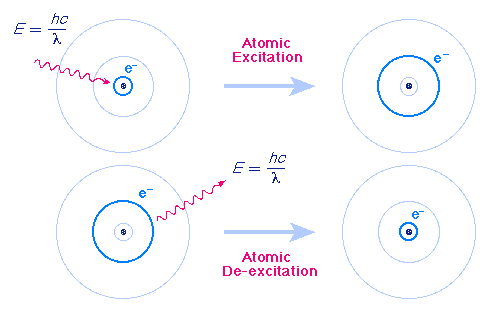
Danish physicist
Niels Bohr (1885-1962) (Detail) won the Nobel prize in physics for his
model of the hydrogen atom, in which the electron occupied
discrete energy levels, or orbits, around the nucleus, and radiated or
absorbed energy only when moving between energy levels, whereas
classical theory predicted that an orbiting electron (a moving charge)
should radiates energy continuously. This model of circular orbits was
able to account for the major series of the hydrogen spectrum, but
it was left to Sommerfeld to explain its fine structure by adding
elliptic orbits to the model, resulting in the Bohr- Sommerfeld atom.
Bohr is commemorated with circular electron orbits on the Swedish stamp
but with an elliptic orbit on the Danish stamp, which also
indicates the quantized nature of the energy transition between orbits.
The stamp showing Bohr and his wife on a garden bench enjoys some
minor fame because the bench only has three legs.
|